Table Of Content
When subjective outcome data (e.g., diagnosis of pneumonia) are being collected during the study period, exposure status should be blinded for the outcome assessors and adjudicators, to prevent responder bias. When previously collected data (i.e., secondary data) are being used, investigators should ideally use outcome definitions that have been validated in previous studies. For example, Hux and others19 validated definitions of diabetes by comparing International Classification of Diseases codes obtained from administrative health care databases in Ontario with diagnostic data from primary care charts. To achieve these advantages, the design characteristics of the case control study must be rigorously applied. The authors outline those characteristics so that readers can avoid misinterpreting cross-sectional studies, case series, and less rigorous reports as case control studies.
Matching Cases and Controls
Clinical expertise is essential for developing exposure and outcome definitions, as well as for understanding the overall clinical context of how the research question fits into the current body of knowledge. Methodologic expertise is critical for ensuring that robust methods are used, to minimize bias and confounding. Similar to the situation for a cohort study, the drug exposures of interest and their definitions should be clearly specified in the methods. Because exposure in a case–control study is determined after the cases have been identified, a period before occurrence of the case, called the “look-back period” or “look-back window”, must be defined. Look-back periods should consider the study hypothesis and thus may vary considerably from one study to another.
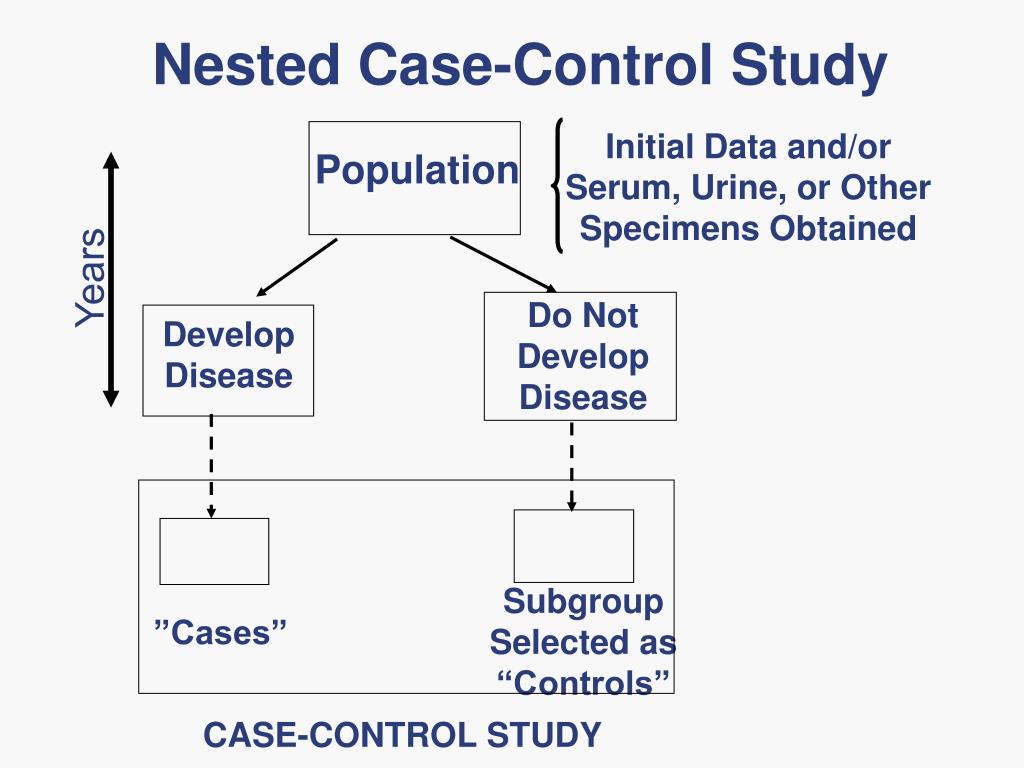
Case-control studies with matching
One also does not have to wait for the development of the outcome – it has already occurred, thus shortening the study process. When designing a case-control study, the researcher must find an appropriate control group. Ideally, the case group (those with the outcome) and the control group (those without the outcome) will have almost the same characteristics, such as age, gender, overall health status, and other factors. If, for example, our cases of Kaposi's sarcoma came from across the country but our controls were only chosen from a small community in northern latitudes where people rarely go outside or get sunburns, asking about sunburn may not be a valid exposure to investigate.
Uses of Case–Control Studies for Therapeutic Effectiveness
The controls should have similar characteristics (i.e., age, sex, demographic, health status) to the cases to mitigate the effects of confounding variables. Finally, case-control studies, like cohort studies, are observational in nature, andauthors who conduct and report such studies should follow the Strengthening the Reporting ofObservational Studies in Epidemiology (STROBE) guidelines. Because of these advantages, case-control studies are commonly used as one of the first studies to build evidence of an association between exposure and an event or disease. The authors conducted a case-control study to study the association between melanoma and tanning.

Data Sources
One of the most important things each party provides is helping identify correct controls for the cases. Matching the controls across a spectrum of factors outside of the elements of interest take input from nurses, pharmacists, social workers, physicians, demographers, and more. Failure for adequate selection of controls can lead to invalid study conclusions and invalidate the entire study. The major method for analyzing results in case-control studies is the odds ratio (OR). The odds ratio is the odds of having a disease (or outcome) with the exposure versus the odds of having the disease without the exposure. The most straightforward way to calculate the odds ratio is with a 2 by 2 table divided by exposure and disease status (see below).
An odds ratio is the ratio of the odds of an exposure in the case group to the odds of an exposure in the control group. A confidence interval that includes 1.0 means that the association between the exposure and outcome could have been found by chance alone and that the association is not statistically significant. Case-control studies cannot provide any information about the incidence or prevalence of a disease because no measurements are made in a population based sample.
Beneficial for studying rare diseases
Only the case-control pairs (A0,A′) with discordant exposure values (i.e., (1,0) or (0,1)) are used. Under the stated sampling schemes and assumptions, the respective estimands are identified by the ratio of discordant pairs. The counterfactual framework offers a language rich enough to articulate a wide variety of causal claims that can be expressed as what-if statements [1]. Another, albeit closely related, approach to causal inference is target trial emulation, an explicit effort to mitigate departures from a study (the ‘target trial’) that, if carried out, would enable one to readily answer the causal what-if question of interest [2]. While it may be too impractical or unethical to implement, making explicit what a target trial looks like has particular value in communicating the inferential goal and offers a reference against which to compare studies that have been or are to be conducted.
COHORT STUDIES
Pigment epithelium-derived factor (PEDF) represses the glucose transporter 1 (GLUT1) mRNA expression and may be ... - Nature.com
Pigment epithelium-derived factor (PEDF) represses the glucose transporter 1 (GLUT mRNA expression and may be ....
Posted: Tue, 05 Dec 2023 08:00:00 GMT [source]
The counterfactual framework and emulation approach have become increasingly popular in observational cohort studies. A notable exception is given by Dickerman et al. [3], who recently outlined an application of trial emulation with case-control designs to statin use and colorectal cancer. It is our hope that a better understanding of the case-control study design and how it differs from other observational studies will promote improved quality in future reporting within the neurosurgical community. Additionally, a better understanding of the case-control study design will enable the clinician to be more critical when interpreting the significance of reported results. Researchers in case-control studies start with a population of people known to have the target disease instead of following a population and waiting to see who develops it. This enables researchers to identify current cases and enroll a sufficient number of patients with a particular rare disease.
The case group would consist of all those patients at the hospital who developed post-operative endophthalmitis during a pre-defined period. You can cite our article (APA Style) or take a deep dive into the articles below. This would allow you to observe whether the people exposed to the chemical had more instances of mesothelioma than those who weren’t exposed. Compare your paper to billions of pages and articles with Scribbr’s Turnitin-powered plagiarism checker.
A population-scale temporal case–control evaluation of COVID-19 disease phenotype and related outcome rates in ... - Nature.com
A population-scale temporal case–control evaluation of COVID-19 disease phenotype and related outcome rates in ....
Posted: Tue, 25 Jul 2023 07:00:00 GMT [source]
They look into the past to find clues, like habits or experiences, that are different between the two groups. This study would be retrospective in that the former lifeguards would be asked to recall which type of sunscreen they used on their face and approximately how often. This could be either a matched or unmatched study, but efforts would need to be made to ensure that the former lifeguards are of the same average age, and lifeguarded for a similar number of seasons and amount of time per season. The odds ratio tells us how strongly the exposure is related to the disease state. An odds ratio of greater than one implies the disease is more likely with exposure.
If care is taken with definitions, selection of controls, and reducing the potential for bias, case-control studies can generate valuable information. One should not use the word case-control study for a randomised controlled trial (even though you have a control group in the study). For a study to be classified as a case-control study, the study should be an observational study and the participants should be recruited based on their outcome status (some have the disease and some do not).
In the hierarchy of study designs used to produce evidence-based guidelines, the role of case-control studies has varied. The level of evidence designation for study designs is a hierarchical classification reflective of the inherent potential for bias that may skew the results that may be introduced by the study design. Because most neurosurgical conditions are rare in the general population and may have long latency periods, this can often be an ideal study choice for neurosurgical clinical research. In a case-control study, on the other hand, the cases, those patients with the outcome of interest, are located and matched, sometimes quite easily, with similar patients, but without the outcome of interest.

No comments:
Post a Comment